Avda. Francisco Vallés, 8 47151
Boecillo (Valladolid) - Spain
(+34) 983 54 98 96
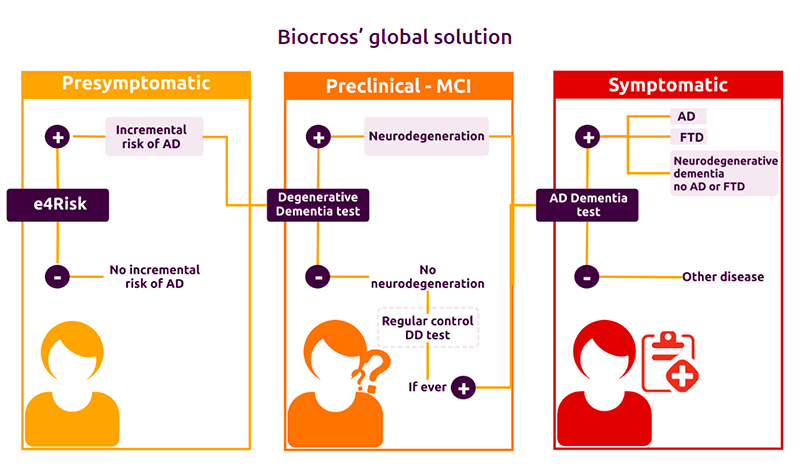
We provide neurologists with an integrated diagnostic solution that can be applied to any stage of the disease, including the earliest, asymptomatic phase.
Our test is designed to discriminate between different forms of dementia to identify and correctly classify patients with Alzheimer’s disease.
Our novel diagnostic approach is made possible by simultaneous analysis of a combination of distinct biomarker types.
We offer cost-effective solutions that can be easily incorporated into hospital routines.
Biocross’ activity is centered firmly on R&D; the company has positioned itself as a developer of diagnostic systems for neurodegenerative diseases to be marketed by a strategic partner in the in vitro diagnostics or pharmaceutical sector.
Our business model is designed to secure the value of Biocross’ intellectual property and know-how. Biocross is focused on generating a product pipeline to offer investors great benefits with reduced risks and the possibility of near-term revenue generation.
The development of strategic partnerships and alliances represents a key challenge for Biocross. The development of strategic partnerships and alliances represents a key challenge for Biocross, and is of the utmost importance given the continuous changes occuring within the field of neurology.

At Biocross, know-how and intellectual property are among our most valuable assets. Thanks to our many years of research, our patents enable us to go where others cannot on the quest to develop innovative technologies.
We can guarantee the quality of our work and provide the documentation and support required to obtain the necessary licensing from health authorities to ensure our products reach the market./p>
Biocross seeks partners for out-licensing opportunities to expand our in vitro diagnostic tests in the field of neurology./p>
Do not hesitate to contact us if you think your business could benefit from licensing any of our intellectual property.

Biocross offers its customers high quality products, services and information. Biocross is certified as meeting the requirements of ISO 9001: 2015 and ISO 13485: 2016 for the activities of design, development and production of in vitro diagnostic (IVD) medical devices. These certifications assure that Biocross has established effective and reliable processes for the development, manufacturing, distribution and customer service of IVD medical devices. With these certifications, we establish a commitment with our clients, with our business and with all those who depend on and benefit from the use of Biocross´ products.
Likewise, Biocross has the installation license that allow us to manufacture IVD medical devices with CE marking. This certification mark guarantees that the products meets the essential requirements of European legislation on health, safety and environmental protection, as per European Directive 98/79/EC and its and RD 1662/2000, its Spanish equivalent.
Quality Policy
BIOCROSS is dedicated to improving healthcare by providing high quality, safe, effective diagnostic products and ensuring compliance.
The Quality Policy is achieved through a commitment to quality and the continuing effectiveness of the Quality System to meet customer and regulatory requirements.
Management demonstrates its commitment to the Quality Policy and legal requirements by ensuring that Quality Policy is understood, implemented, communicated, and maintained at all levels of the organization./p> p>The Quality Policy is deployed throughout the organization via the Quality System, operating procedures, quality system training, and management’s reinforcement of quality principles and practices.
Employees support the Quality Policy through compliant execution of quality system requirements; and as part of every employee’s responsibility is the continual improvement of quality systems and product quality to increase customer satisfaction.






